Useful information for prescribers
Answering your questions on prescribing qualifications, standards and practice.
Which nursing and midwifery professionals can prescribe?
Only registered nurses (level 1), midwives and those who have qualified as specialist community public health nurses (SCPHN) who have successfully completed an NMC approved prescribing programme and have their qualification recorded on our register are eligible to prescribe. SCPHNs are registered level one nurses or midwives who have successfully completed a post-registration SCPHN programme approved by us and are on the SCPHN part of the NMC register.
Nursing associates and registered nurses (level 2) are not eligible to undertake a prescribing qualification so are not able to prescribe.
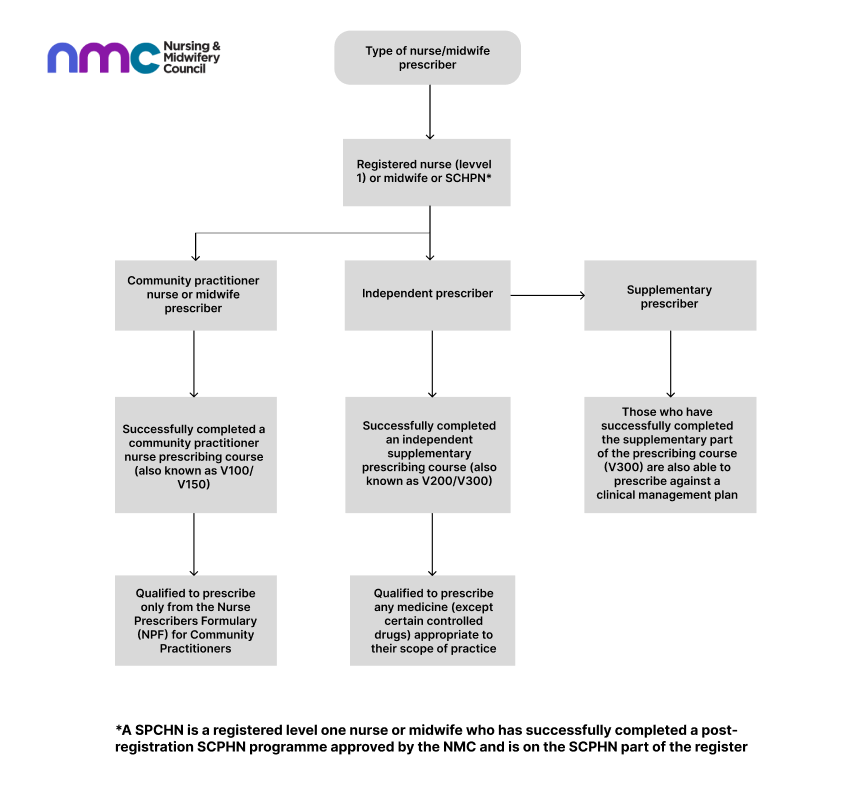
What are the different categories of prescribers?
There are two categories of nurse or midwife prescribers: community nurse or midwife prescribers, and independent and supplementary nurse or midwife prescribers. The diagram below illustrates the categories in more detail.
What are the standards for nurse and midwife prescribers?
All nurse and midwife prescribers (as described above) are educated in accordance with our Standards for prescribing programmes (NMC, 2018). Once qualified and registered, we expect all nurse and midwife prescribers to adhere to the Royal Pharmaceutical Society’s (RPS) Competency Framework for all Prescribers (published 2016, updated 2021) as the standards for safe and effective prescribing practice and the NMC Code which sets out the standards of practice and behaviour we expect of all our registered nursing and midwifery professionals.
We can take action through our fitness to practise processes where professionals on our register fail to uphold professional standards or practise outside relevant guidance that poses a risk to patient safety or public confidence in the nursing and midwifery professions. Further information about fitness to practise and how our processes work.
Using prescribing responsibilities obtained through a NMC prescribing qualification (V100/V150/V200/V300) while working as a Physician Associate (PA) or Anaesthesia Associate (AA)
The Department of Health and Social Care has confirmed that individuals working in PA and AA roles cannot lawfully prescribe using prescribing rights from another regulated role.
Prescribing rights are specific to the regulated profession in which they were granted and aren’t transferable. For example, if you gained prescribing rights as a nurse or doctor, you cannot use them while working as a PA or AA.
From 13 December 2024, healthcare professionals working in designated PA or AA roles should not prescribe medicines, even if they hold prescribing rights from a previous profession or have previously been authorised to prescribe by their employer.
What nursing or midwifery prescribers need to consider when deciding whether to prescribe online or remotely
Although it is not our role to issue clinical guidance, or guidance relating to individual named medicines, all nurse or midwife prescribers must consider the information set out below.
Prescribing at a distance carries different opportunities and risks to prescribing in face-to-face settings.
The RPS competency framework contains several supporting statements related to the prescriber role which describe the activity or outcome that the prescriber should actively and routinely demonstrate including when prescribing online or remotely. These include, but are not limited to, that the professional:
- Undertakes the consultation in an appropriate setting (competency 1.1)
- Demonstrates good consultation skills and builds rapport with the patient/carer (competency 1.5)
- Takes and documents an appropriate medical, psychosocial and medication history including allergies and intolerances. (competency 1.6)
- Undertakes and documents an appropriate clinical assessment (competency 1.7)
- Identifies and addresses potential vulnerabilities that may be causing the patient/carer to seek treatment (competency 1.8)
- Accesses, critically evaluates, and uses reliable and validated sources of information (competency 2.7)
- Builds a relationship which encourages appropriate prescribing and not the expectation that a prescription will be supplied (competency 3.5)
- Follows appropriate safeguards if prescribing medicines that are unlicensed, off-label, or outside standard practice (competency 4.12)
- Establishes and maintains a plan for reviewing the patient's treatment (competency 6.1)
- Identifies and minimises potential risks associated with prescribing via remote methods (competency 7.3)
- Prescribes within own scope of practice, and recognises the limits of own knowledge and skill (competency 7.12)
All prescribers have a professional responsibility to ensure medicines are only supplied where they are clinically appropriate and where they have enough knowledge of the intended recipient’s health and their full medical and prescribing history. Prescribers need to assess the suitability of their consultation and prescribing methods and recognise that there are medicines and areas of practice where it would not be suitable to prescribe remotely or where additional safeguards need to be in place to ensure medicines are supplied safely and appropriately.
Can nurse and midwife prescribers prescribe medicines remotely?
Yes. However, not all medicines are suitable to be prescribed by remote methods such as telephone, email, online video or communication via a third party.
All prescribers must take individual responsibility for their prescribing decisions and should recognise that there are certain areas of practice where remote prescribing is unlikely to be suitable. Examples include, but are not limited to, medicines likely to be subject to misuse or abuse, high risk medicines that need extra safeguards, where there is not enough information about the recipient’s health, medical and prescribing history to safely prescribe (e.g. no access to medical records or the mode of consultation is unsuitable to meet the individual’s needs) or where there needs to be a physical examination of the person in order to ensure safe prescribing.
All prescribers are responsible for the prescriptions they sign, and they must be prepared be prepared to explain and justify their decisions and actions when prescribing, administering and managing medicines or declining to prescribe.
Can nurses and midwives prescribe for non-surgical cosmetic procedures remotely?
The non-surgical cosmetic procedures landscape comprises of a vast range of procedures, techniques, products and services, with wide variation in complexity and invasiveness.
The Health and Care Act 2022 defines a ‘cosmetic procedure’ as a procedure, other than a surgical or dental procedure, that is or may be carried out for cosmetic purposes; and the reference to a procedure includes—
(a) the injection of a substance;
(b) the application of a substance that is capable of penetrating into or through the epidermis;
(c) the insertion of needles into the skin;
(d) the placing of threads under the skin;
(e) the application of light, electricity, cold or heat.
We use a broad definition to define non-surgical cosmetic procedures to mean elective interventions, procedures or treatments that are carried out with the primary purpose of changing or enhancing a person’s physical appearance for cosmetic reasons, rather than for medical necessity.
Following a review of our position on the remote prescribing of non-surgical cosmetic products in 2024 and to support safe prescribing decisions, effective from 1 June 2025 independent nurse and midwife prescribers must carry out a face-to-face consultation and undertake and document an appropriate clinical assessment of the intended recipient of the medicine before they prescribe any products used for any elective non-surgical cosmetic procedure. It is not appropriate to prescribe medicinal products for these purposes by remote methods such as telephone, email, online, video or communication via a third party. This applies to the initial consultation and any subsequent consultations and follow ups.
This position is consistent with the requirements of the RPS Prescribing Competency Framework and the High level principles for good practice in remote consultations and prescribing which includes the requirement that healthcare professionals make patient safety their first priority and ensure that adequate clinical assessments are always undertaken.
Any nursing or midwifery professional who chooses to provide elective non-surgical cosmetic procedures are expected to adhere to professional standards at all times. This includes making sure they are appropriately educated and trained, competent and have appropriate indemnity cover. The prescriber is responsible for obtaining informed consent from the intended recipient, assessing the suitability of a procedure for each individual and explaining the risks and benefits of a procedure to them.
When a prescriber delegates the administration of a medicine as part of a non-surgical cosmetic procedure they must ensure that the person they delegate to is suitably qualified and be satisfied that they are competent and proficient in administering the medicine and carry out the procedure at suitable premises. Prescribers remain responsible at all times for the overall oversight and care of each individual, including assessing outcomes and managing any adverse incidents or complications.
Can nurses and midwives hold prescription medicines as stock?
The Human Medicines Regulations 2012 only permits doctors and dentists to hold stock of prescription only medicines (where medicines have not been dispensed to a named patient by a pharmacist). The Medicines and Healthcare products Regulatory Agency (MHRA) has advised nurse and midwife independent prescribers are not eligible to be supplied with prescription medicines as stock. A Patient Specific Direction (PSD) is the legal method of prescribing, including for non-surgical cosmetic procedures and when prescribers delegate the administration of a prescription only medicine.
In Scotland, Healthcare Improvement Scotland (HIS) allows nurse independent prescribers, owning HIS-registered clinics to hold prescription medicines as stock on their premises.
What nursing and midwifery professionals involved in administration of medicines need to consider
Any nurse, midwife or nursing associate administering medicines that have been prescribed by another person (including when the prescribing has been done remotely) has a responsibility under the Code to ensure that they’re satisfied that the RPS competency framework has been followed, and patient safety is not at risk.
This means any nursing or midwifery professional must refuse if asked to supply or administer any medicinal product if they have concerns that the prescribing has not been carried out appropriately and in line with the requirements of the Code, the RPS Competency Framework or other applicable standards and guidance.
If a nurse, midwife or nursing associate is concerned that patient or public safety is being put at risk, or they are being asked to work outside of the requirements of the Code or any other national standards, they are empowered under the Code to raise their concerns (see sections 16.1 and 16.3 of the Code for more details).
High level principles for good practice in remote consultations and prescribing
In November 2019 healthcare organisations (including regulators, royal colleges and faculties) issued a set of principles to help protect patient safety and welfare when accessing potentially harmful medications online or over the phone.
The jointly-agreed High level principles for good practice in remote consultations and prescribing set out the good practice expected of healthcare professionals when prescribing medication online.
The ten principles, which are underpinned by existing standards and guidance, include that healthcare professionals are expected to:
- understand how to identify vulnerable patients and take appropriate steps to protect them
- carry out clinical assessments and medical record checks to ensure medication is safe and appropriate
- raise concerns when adequate patient safeguards aren’t in place.
Other useful sources of information
- Nursing and Midwifery Council
- The General Medical Council
- Remote consultations flowchart offers helpful guidance on the factors to consider around consulting remotely
- Good practice in proposing, prescribing, providing and managing medicines and devices
- Cosmetic interventions guidance
- The General Dental Council
- The General Pharmaceutical Council
- Royal Pharmaceutical Society
- Advertising Standards Authority (ASA)
- Medicines & Healthcare products Regulatory Agency
- Joint Council for Cosmetic Practitioners
- British Association of Medical Aesthetic Nurses
- Save Face
- Risks of buying medicines over the internet